B.O.B’s Journey So Far
With our patent for the technology behind ACT Medical’s novel device, B.O.B, now officially granted, it felt like the right time to pause, look back, and reflect on how far we’ve come — and the people and partnerships who’ve helped us get here.
As many of you will know, ACT’s story began when Joe, our founder, was studying at university. Two of his friends were stabbed, and as a result, he started wondering if there was something he could design to help first responders treat those kinds of injuries faster. That idea became the REACT device, and in 2021, Joe was awarded the first-ever Global Medical James Dyson Award. That recognition, and the support that came with it, marked the start of ACT Medical.
Our mission was clear from day one: to reduce deaths from penetrating trauma. The technology has evolved a long way since those early prototypes, but the mission, and the drive behind it, hasn’t changed.
Back to the Drawing Board
REACT was originally a university project, so it came with the usual constraints of that environment. It featured a reusable handle and a single-use balloon component designed to apply pressure inside a wound and control bleeding before a patient reached hospital care.
When ACT Medical was founded, we wanted to build on that principle and take it further. To do that properly, we started by talking directly to the people who’d actually use it.
It’s easy as engineers to design what we think works, but this time we wanted to get it right from the ground up. Through interviews and focus groups with first responders, we learned what really mattered: the device needed to be affordable, widely available, and incredibly simple to use.
As one paramedic told us, “In pre-hospital care, every second counts — and complexity costs time.” That sentiment is summed up by a phrase we’ve heard again and again: “Keep It Simple, Stupid.”
That gave us our design direction: find a purely mechanical way to apply internal pressure, no motors, no fluids, keeping cost, weight, and complexity to an absolute minimum.
The Hive Mind
With a clear brief to focus on mechanical solutions simple enough to work reliably in pre-hospital scenarios, we partnered with TTP plc — a Cambridge-based consultancy known for delivering creative solutions to hard problems. We trusted TTP to blend the right level of deep scientific analysis, practical medical device engineering and user-centred design to hit this unique target.
Working closely with TTP in collaborative workshops, we combined rapid fail-fast prototyping with rigorous engineering modelling and structured usability evaluations to test each concept against demanding clinical, safety, and manufacturability requirements. This process allowed us to explore the full breadth of viable routes to delivering internal pressure safely and effectively, but did so quickly and efficiently.
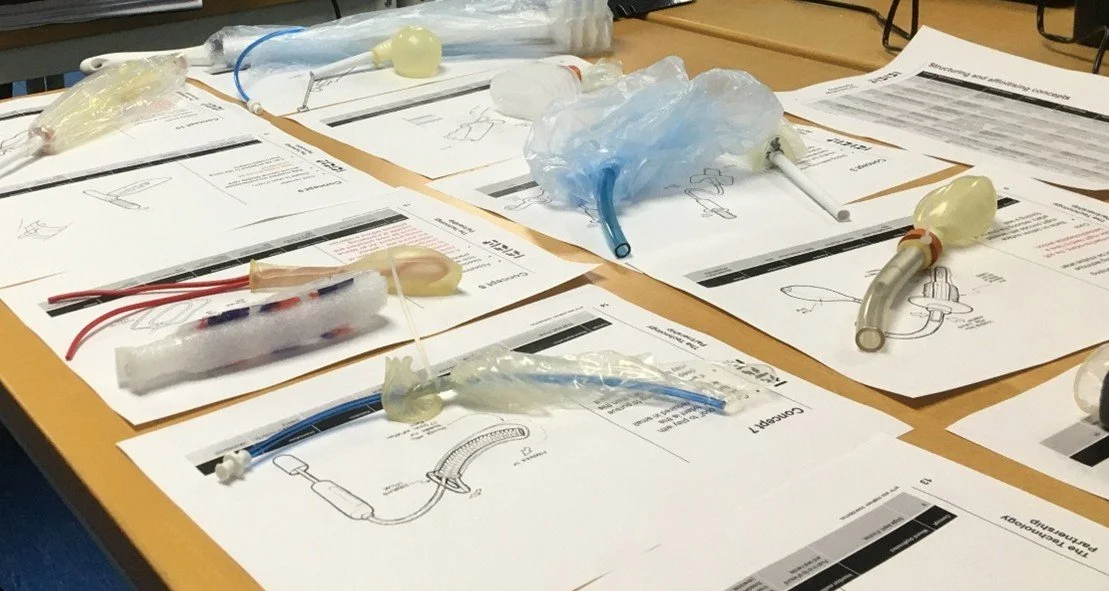
Over 40 concept ideas were mocked up, from balloons to bottlebrush-style mechanisms, with quick, hands-on tests to see what worked best. After several rounds of iteration and evaluation, we narrowed those down to seven promising concepts, and then to three that performed well when tested with perfused animal tissue in TTP’s labs.
TTP then hosted a formative user study with six paramedics, each with different backgrounds and experience levels, to see how they interacted with the prototypes. The simulated pre-hospital trauma scenarios generated detailed, nuanced insights into usability and potential failure points. TTP analysed these findings and turned them into actionable recommendations.
We combined those insights with data from benchtop testing, intellectual property reviews, and clinical input to identify one standout concept — the one that would become B.O.B.
What made this direction particularly interesting was that the chosen concept came with a greater technical challenge. The concept relied on the development of a novel material, something that didn’t exist off the shelf and would need to deliver very specific mechanical behaviour to achieve the performance required. Through early feasibility work and the generation of an in-silico engineering model, TTP demonstrated that it was theoretically possible to develop such a material and identified a potential manufacturer capable of meeting those requirements.
Choosing it was a brave move: higher technical risk up front, but the potential for a design that was dramatically simpler, more intuitive, and safer for first responders. In the end, that trade-off felt true to ACT’s mission and our design philosophy — if the material could be developed, it would enable a level of simplicity that could make a real difference in the field. With that, the patent was filed.
It works!
Once we had our concept, we needed to prove that it actually worked — both mechanically and clinically.
Working with subject-matter experts, we developed a novel material that could serve as B.O.B’s core. We then verified through benchtop testing that this material delivered the performance required to generate safe, reliable internal pressure across a range of wound sizes.
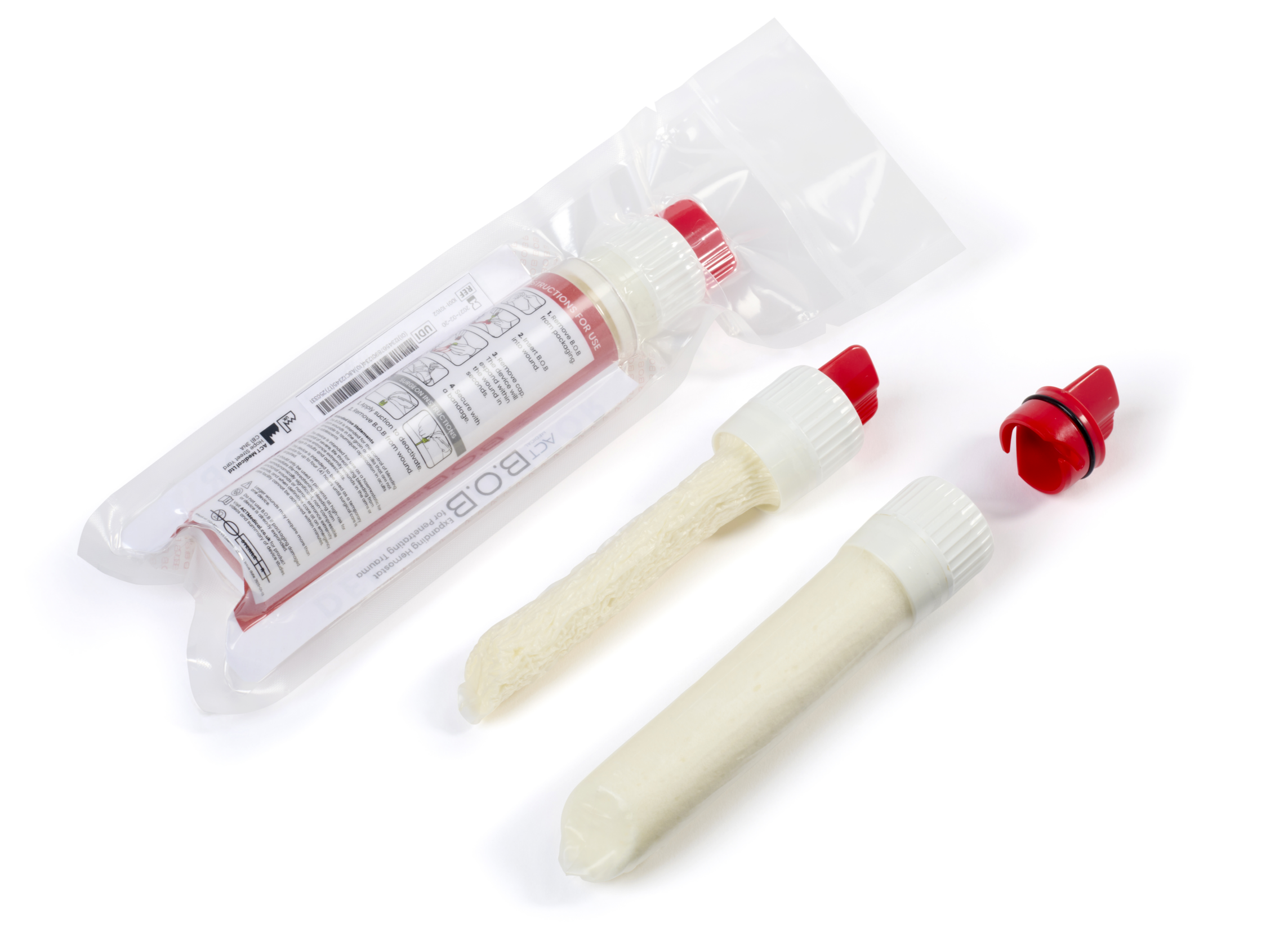
Alongside this, our team developed a minimum viable product (MVP) that looked and functioned like the intended final version. Using insights from usability engineers and mechanical designers, we refined the design into something intuitive:
Insert into the wound.
Remove the cap to activate.
Simple, effective, and manufacturable.
With TTP’s help we then tested this version with ten U.S.-based first responders in Boston. This round surfaced finer details around users’ comprehension, feedback cues, and the communication needed for clear instructions for use. Even with untrained participants, users could deploy B.O.B quickly and effectively — a huge validation of the device’s intuitive design.
At the same time, we carried out our first in-vivo pre-clinical study, which demonstrated that direct internal pressure could reliably stop bleeding from junctional wounds within three minutes — in 100% of cases. No adverse effects were observed, and blood flow beyond the wound remained stable.
That was the proof we needed: B.O.B works.
Designing for Scale
Once we’d proven that B.O.B could stop bleeding effectively, the next challenge was scaling up. Moving from a successful prototype to a device that can be built, distributed, and relied upon in real emergencies is no small feat, and it required a different kind of thinking.
To help us make that leap, we partnered with Springboard, a fellow Cambridge-based company and part of the Sanner Group. With deep expertise in manufacturing and strong connections to U.S. production partners, they were the perfect team to help us translate innovation into something ready for the real world.
Their support went far beyond optimising parts and processes. Alongside the manufacturing work, Springboard also carried out targeted usability design improvements to address several user errors observed in our earlier formative study. By refining affordances and simplifying cues, they helped ensure that the scaled design would be intuitive and reliable in the field.
On the manufacturing side the goal was clear: make B.O.B as simple and affordable to produce as it is to use. Together, we revisited every component and assembly step, looking for ways to reduce cost, complexity, and build time.
The result? A halved cost of goods, two-thirds less assembly time, and a design that can now be manufactured right here in our ISO 13485-aligned build space — hundreds of units at a time.
What’s Next?
B.O.B’s journey so far is a true testament to Cambridge’s medical device community. Having our base in Hope Street Yard means we’re surrounded by brilliant minds and companies who’ve played a huge role in shaping, as our users have said, the “genius” solution that is B.O.B.
Over the next year, we’ll be working with U.S.-based manufacturers to establish a validated production line and begin verification and validation testing for FDA submission. Once approved, B.O.B will finally be in the hands of first responders — where it belongs.
And that’s when the real impact begins.
If you’re interested in supporting ACT’s next chapter, get in touch at hello@actmedical.co.uk to learn more.
ACT Medical’s award-winning medical device concept aims to save the lives of trauma victims by stemming bleeding from knife and gunshot wounds. Follow ACT Medical on LinkedIn for latest updates or email hello@actmedical.co.uk to speak to us directly.